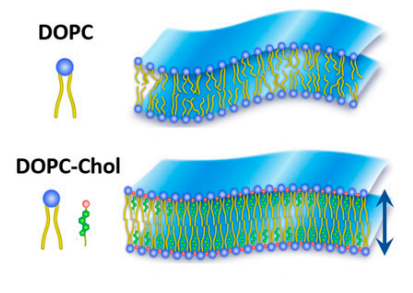
"Cholesterol is known to promote tighter molecular packing in cell membranes, but reports about how it stiffens membranes have been so conflicting," said Ashkar, who is a faculty member in the Virginia Tech College of Science. "In this work, we show that, at the nanoscale level, cholesterol indeed causes membrane stiffening, as predicted by physical laws. These findings affect our understanding of the biological function of cholesterol and its role in health and disease."
According to the study, cell membranes are thin layers of fatty molecules that define cell boundaries and regulate various biological functions, including how viruses spread and how cells divide. To enable such functions, membranes should be able to bend and permit shape changes. This bending propensity is determined by how packed the molecular building blocks are; tighter packing results in stiffer membranes that cannot bend so easily, Ashkar added.
At the molecular level, cholesterol possesses a slick and rigid structure. When it interacts with our cell membranes, it jams itself right in between lipids, which results in a more densely packed membrane. According to structure-property relations, this would naturally result in a stiffer membrane.
Yet, for the past 10 or so years, physicists and biologists have assumed that cholesterol had nearly no effect on the stiffness of membranes formed of cis-unsaturated lipids, a common type of lipid found in our cells, despite its well-documented effect on lipid packing.
"It defied our understanding of what cholesterol does to cell membranes," Ashkar said. "It also contradicts standard structure-property relationships in self-assembled materials."
Ashkar found a clear case of how soft materials can "apparently" exhibit different properties, depending on the parameters of the observation method. She found that over short length and time scales over which important signaling events occur—we're talking nanometers and nanoseconds—the added cholesterol induces membrane stiffening that one would expect.
from paper
Chol plays an essential regulatory function in many biomembrane processes (3), including passive permeation (4), protein and enzyme activity (5, 6), and the formation of raft-like domains (7) associated with cell signaling and intracellular trafficking. It is also directly implicated in viral infections (8), including influenza (9), HIV (10), and, recently, coronavirus (11). Deviations from normal Chol levels disrupt membrane functions and result in impaired immune responses and numerous other health anomalies (12–14).
While early studies performed on single-component lipid membranes exhibited the expected dependence of the bending rigidity as a function of membrane thickness and packing density (22), later studies on Cholcontaining membranes showed striking deviations from this behavior, which, surprisingly, depended on the level of lipid acylchain unsaturation (23, 24). Given the lipid diversity in cell membranes and the significance of mesoscale bending energetics in viral budding (25) and membrane–protein interactions (26)
reference
Saptarshi Chakraborty et al. How cholesterol stiffens unsaturated lipid membranes, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.2004807117
3. F. R. Maxfield, G. van Meer, Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 22, 422–429 (2010).
4. E. Corvera, O. G. Mouritsen, M. A. Singer, M. J. Zuckermann, The permeability and the effect of acyl-chain length for phospholipid bilayers containing cholesterol: Theory and experiment. Biochim. Biophys. Acta 1107, 261–270 (1992).
5. F. J. M. de Meyer, J. M. Rodgers, T. F. Willems, B. Smit, Molecular simulation of the effect of cholesterol on lipid-mediated protein-protein interactions. Biophys. J. 99, 3629–3638 (2010).
6. F. Cornelius, Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry 40, 8842–8851 (2001).
7. J. Bernardino de la Serna, J. Perez-Gil, A. C. Simonsen, L. A. Bagatolli, Cholesterol rules: Direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 279, 40715–40722 (2004).
8. Y. Deng, Z. A. Almsherqi, M. M. L. Ng, S. D. Kohlwein, Do viruses subvert cholesterol homeostasis to induce host cubic membranes? Trends Cell Biol. 20, 371–379 (2010).
9. X. Sun, G. R. Whittaker, Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 77, 12543–12551 (2003).
10. V. R. Prasad, M. I. Bukrinsky, New clues to understanding HIV nonprogressors: Low cholesterol blocks HIV trans infection. MBio 5, e01396-14 (2014).
11. G. Meher, S. Bhattacharjya, H. Chakraborty, Membrane cholesterol modulates oligomeric status and peptide-membrane interaction of severe acute respiratory syndrome coronavirus fusion peptide. J. Phys. Chem. B 123, 10654–10662 (2019).
12. K. Matsuzaki, K. Sugishita, N. Fujii, K. Miyajima, Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry 34, 3423–3429 (1995).
13. A. Khondker et al., Membrane cholesterol reduces polymyxin B nephrotoxicity in renal membrane analogs. Biophys. J. 113, 2016–2028 (2017).
14. A. J. McHenry, M. F. M. Sciacca, J. R. Brender, A. Ramamoorthy, Does cholesterol suppress the antimicrobial peptide induced disruption of lipid raft containing membranes? Biochim. Biophys. Acta 1818, 3019–3024 (2012).
22. D. Boal, Mechanics of the Cell (Cambridge University Press, Cambridge, UK, ed. 2, 2002).
23. J. Pan, S. Tristram-Nagle, J. F. Nagle, Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E 80, 021931 (2009).
24. R. S. Gracià, N. Bezlyepkina, R. L. Knorr, R. Lipowsky, R. Dimova, Effect of cholesterol on the rigidity of saturated and unsaturated membranes: Fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 6, 1472–1482 (2010).
25. B. J. Reynwar et al., Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 447, 461–464 (2007).
26. M. F. Brown, Soft matter in lipid-protein interactions. Annu. Rev. Biophys. 46, 379–410 (2017). 27. M. Dokt