All biological processes are in some way pH-dependent. Human bodies and those of other organisms need to maintain specific and constant pH regulation in order to function. Changes in pH can have serious biological consequences—or serious benefits.
Cellulosomes are extracellular complexes consisting of multiple enzymes, which are associated with the cell's surface. Within the cellulosome cellular structure, the protein molecules dockerin and cohesin were the focus of this study.
"Cellulosomes are complex nanomachines in nature and have great values in biofuel production and biotechnology. This study is an example of the complexity and diversity of cellulosomes,"
Changes in pH have previously been shown to result in "on-off" switches within protein functions, many of which occur naturally and are essential for life processes. Biotechnical innovations can utilize this relevant phenomenon to develop sensors or switches using biomolecules that are pH-dependent.
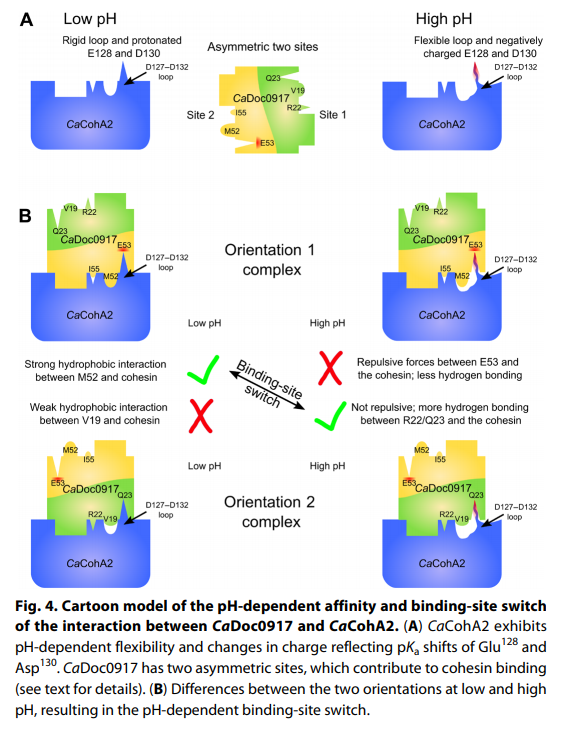
The latest discovery, on the cellulosome assembly of the bacterium Clostridium acetobutylicum, takes this prospect further by switching between two functional sites, rather than simply on or off. This opens additional possibilities.
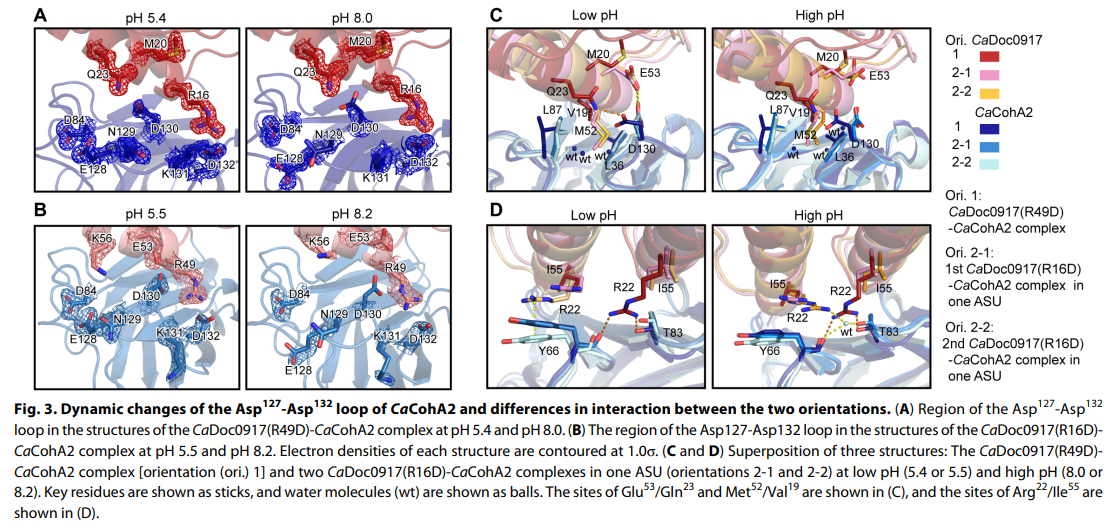
Researchers found that changing the pH from 4.8 to 7.5 resulted in the cohesin-binding sites on the dockerin molecule switching from one site to the other.
Nuclear magnetic resonance (NMR) and isothermal titration calorimetry (ITC) were used to describe the distinct features of this interaction.
Researchers additionally noted that the affinity, or the attraction between the molecules, was found to change along with the pH.
reference
"Discovery and mechanism of a pH-dependent dual-binding-site switch in the interaction of a pair of protein modules" Science Advances (2020). DOI: 10.1126/sciadv.abd7182
'ect' 카테고리의 다른 글
| do fish school reduce energy cost of swimming? (0) | 2020.10.27 |
|---|---|
| pericytes들을 연결해주는 cell structure (0) | 2020.08.13 |
| how to overcome coffee ring effect (0) | 2020.08.13 |
| 세포 스트레스로 인한 리보솜 충돌에 따른 cell fate (0) | 2020.08.06 |


